Covalent radius: It is half of the distance between the nuclei of two like atoms bonded together by a single bond. Therefore covalent radius of carbon in a compound having C - C single bond can be determined by dividing bond length by 2, that is Rc = (c-c)/2 Thus, C-C = 2rc or rc + rc. Covalent radius The atomic radius of an atom (e.g. Carbon), determined in a covalent compound by a technique which can be used only for structures in which that atom (carbon) is covalently bonded. Measurement of Radius. There are several methods that can be used to determine radii of atoms and ions: Nonpolar atomic radii: The radius of an atom is derived from the bond lengths within nonpolar molecules; one-half the distance between the nuclei of two atoms within a covalent bond. Van der Waals radius: The radius of an atom is determined by collision with other atoms. Other articles where Covalent radius is discussed: atomic radius: radius is designated as a covalent radius.
Why is van der Waals radius greater than covalent radius for covalent compounds?
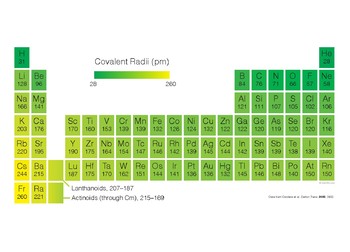
Covalent Radii
1 Answer
Explanation:
The van der Waals radius and the covalent radius actually deal with two different situations.
The former is used when dealing with atoms that are not bonded, and the latter is used for atoms that are covalently bonded.
The main reason for why the van der Waals radius is greater than the covalent radius is that it does not take into account overlap.
To determine the van der Waals radius of an element, you need to get two atoms that belong to that element and get them as close as possible to each other.

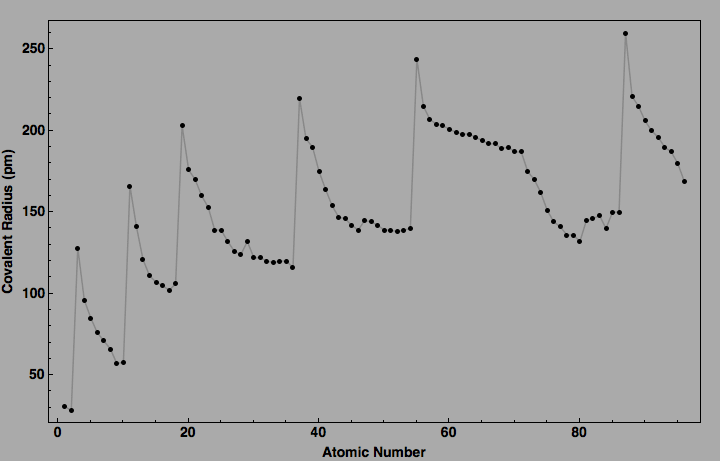
At this point, you measure the internuclear distance, i.e. the distance between their two nuclei, and divide it by 2.
Remember, these atoms are not bonded, they are just very, very close to each other - as close as possible - but not overlapping!
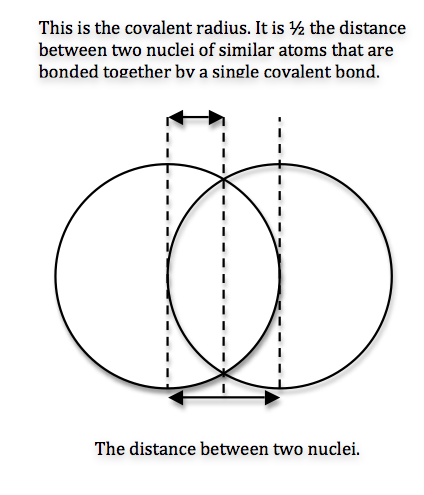
For the same element, you determine the covalent radius by looking at the diatomic molecule that forms when those two atoms form a covalent bond.
When two atoms form a covalent bond, their electron clouds overlap. In essence, that is what a covalent bond really is - an overlap between orbitals.
Since a part of their electron clouds overlap, the atoms will be a little closer to each other. This is why, when you measure the internuclear distance between the two and divide it by 2 you get a smaller value.
Here's one more image of how the van der Waals and covalent radii look for the same element
Covalent Radii Of Hydrogen
The overlap that exists between the two electron clouds is what causes the covalent radius to be smaller than the van der Waals radius.
Related questions

Comments are closed.